Exploring Color Changes
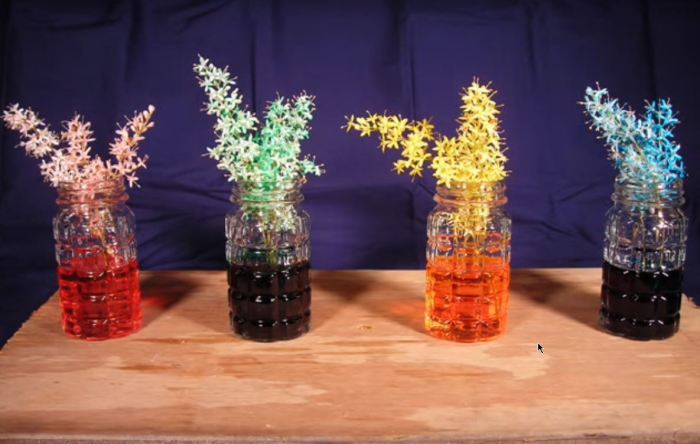
Bleach and food coloring experiment – The reaction between bleach (sodium hypochlorite) and food coloring offers a captivating demonstration of chemical kinetics. The vibrant hues of the food coloring dramatically fade as the bleach oxidizes the dye molecules, altering their chemical structure and thus their ability to absorb and reflect light. This experiment allows for a visual exploration of reaction rates and their dependence on factors like concentration and temperature.The color change observed is a result of a redox reaction.
Bleach, a strong oxidizing agent, donates oxygen atoms to the dye molecules. This process alters the conjugated π-electron system within the dye, which is responsible for its color. The disruption of this system leads to a decrease in the dye’s ability to absorb specific wavelengths of light, resulting in a fading or complete loss of color. The specific chemical reactions involved are complex and vary depending on the type of food coloring used, but generally involve the breaking of double bonds within the chromophore (the part of the molecule responsible for color) and the addition of oxygen atoms.
Bleach Concentration and Reaction Rate
Increasing the concentration of bleach significantly accelerates the rate of color change. A higher concentration provides a greater number of hypochlorite ions (OCl⁻), the active oxidizing agents in bleach, leading to more frequent collisions with the dye molecules. For instance, if we compare a 10% bleach solution to a 5% bleach solution reacting with the same amount of food coloring, the 10% solution will cause a much faster and more pronounced fading.
This is because the higher concentration results in a greater probability of successful collisions between bleach and dye molecules, leading to a faster oxidation reaction.
Right, so me and me mate were doing this mega dodgy experiment with bleach and food colouring, proper mental colours it was. To get started, we needed to grab some food colouring, so I checked online what aisle is food coloring in to save time, then it was full steam ahead with the experiment! The results were, like, totally wicked, a right laugh, innit?
Temperature’s Effect on Reaction Rate
Temperature plays a crucial role in the reaction rate. Increasing the temperature increases the kinetic energy of the molecules, leading to more frequent and energetic collisions between the bleach and the dye. These more energetic collisions are more likely to overcome the activation energy barrier of the reaction, thus increasing the reaction rate. For example, a solution at 40°C will show a much faster color change compared to the same solution at room temperature (approximately 25°C).
This is consistent with the Arrhenius equation, which describes the relationship between reaction rate and temperature.
The Arrhenius equation: k = A
exp(-Ea/RT)
where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin.
Variations in Food Coloring

The seemingly simple act of adding food coloring to a bleaching solution reveals a surprising complexity in reaction rates and final outcomes. Factors like the type of food coloring used – liquid versus gel – and the specific color chosen significantly influence the experiment’s results. Understanding these variations provides deeper insight into the chemical interactions at play.Different food colorings exhibit varying degrees of solubility and chemical stability, impacting their reaction with bleach.
This leads to observable differences in the speed and intensity of color change, as well as the final hues produced. The chemical composition of the dyes, specifically their molecular structure and reactivity, are key determinants in these observations.
Liquid Versus Gel Food Coloring Reaction Rates, Bleach and food coloring experiment
Liquid food colorings, typically water-based, tend to disperse more readily in the bleaching solution compared to gel food colorings. This increased solubility often translates to a faster initial reaction rate, resulting in a quicker observable color change. Gel food colorings, containing higher concentrations of dye and thickening agents, often exhibit slower diffusion and therefore slower reaction rates. The thicker consistency of gel food coloring impedes the interaction between the dye molecules and the bleach, delaying the bleaching process.
Imagine dropping a single drop of liquid red food coloring versus a small dollop of red gel food coloring into a clear solution – the liquid would spread and mix significantly faster.
Color Variations and Reaction Outcomes
The specific color of the food coloring also influences the observed changes. Certain colors may react more rapidly or intensely with bleach than others due to the chemical structure of the individual dyes. For instance, some dyes may be more susceptible to oxidation by bleach than others. A vivid blue food coloring might fade more quickly than a less reactive yellow, leading to differences in the final color of the solution.
The intensity of the original color also plays a role; a highly concentrated dye might require a longer time to fully bleach compared to a more dilute solution.
Impact of Chemical Composition
The chemical composition of food coloring directly affects its reaction with bleach. Different dyes have different molecular structures, leading to variations in their reactivity. Some dyes are more stable and resistant to bleaching agents, while others are more readily oxidized or degraded. The presence of specific chemical groups within the dye molecule can significantly influence its susceptibility to bleaching.
For example, dyes containing certain aromatic rings might exhibit different bleaching behavior compared to those with aliphatic structures. These variations in chemical structure and composition ultimately determine the speed and extent of the color change observed during the experiment.
FAQ Section: Bleach And Food Coloring Experiment
Can I use any type of bleach for this experiment?
It’s best to use standard household bleach (sodium hypochlorite). Avoid using other types of bleach as they may produce unpredictable results or pose safety hazards.
What happens if I use too much bleach?
Using excessive bleach can lead to a rapid and potentially uncontrolled reaction, making it difficult to observe the color changes accurately. Start with small amounts and adjust as needed.
What safety precautions are crucial besides wearing gloves?
Always conduct the experiment in a well-ventilated area. Avoid contact with eyes and skin. Supervise children closely during the experiment.
Why do some colors fade faster than others?
Different food colorings have varying chemical compositions and thus react differently with bleach. Some dyes are more susceptible to oxidation than others, resulting in varied fading rates.
